Comparison of Outpatient Nebulized vs Metered Dose Inhaler Terbutaline in Chronic Airflow Obstruction: Material and Methods
Dose Response Study
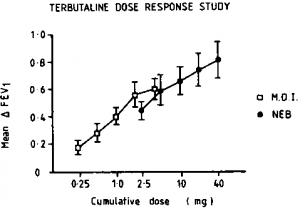
Prior to the trial, the doses of terbutaline administered by MDI and NEB required to produce similar degrees of bronchodilatation were established using separate cumulative dose response curves (Fig 1). Eight of the nine asthmatic patients who went on to participate in the outpatient study were tested on two consecutive days when baseline FEV, differed by less than 10 percent. Therapy with MDI terbutaline 1.25 mg (five puffs) was found to be equipotent with NEB terbutaline 2.5 mg (0.25 ml). These doses were used in the acute response and outpatient studies.
Trial Design
All patients were seen prior to commencement of the trial when the aims of the study were explained and informed consent was obtained. Two practice 6-min walking distance tests (6MWD) were performed and blood was drawn for serum theophylline estimation. The trial was in two parts. Part 1 was an acute-response study where cumulative unit doses of terbutaline MDI and NEB were given until FEV, plateaued (increased by less than 100 ml after two consecutive doses). Part 2 was the outpatient study where unit doses of terbutaline MDI and NEB were taken four times daily for each of two treatment fortnights. The test treatment consisted of unit doses of five puffs of terbutaline or placebo MDI and 0.25 ml terbutaline or placebo solution which was added to 1.75 ml of normal saline solution. The MDI technique was carefully assessed by one physician. It involved placing the mouthpiece between the lips with activation at the beginning of a slow inspiration from residual volume to total lung capacity followed by a breath-hold of 5 to 10 s. Subsequent doses were administered 30 s apart. The nebulizer solution was administered by an Aerfio nebulizer (Biospectrum, Australia) and driven by an Allersearch Vitalair compressed air pump which delivers 8 L/min of air at a maximum pressure of 200 kPa. Although the characteristics of volume output and particle size for this nebulizer with this pump were not determined, the nebulizer, when driven by compressed gas at 6 L/min and at a pressure of 100 kPa, produced a volume output of 0.24 cu mm/min and 95 percent of the particles were between 1.2 and 4.45 micrometers in diameter. combigan eye drops
Patients breathed tidally from a face mask (Aerflo) until visible aerosol production ceased (10-20 min). Patients refrained from using their usual beta-agonist medication unless necessary. The trial was of a double-blind, crossover, placebo-control design. The patients were allocated to one of two treatment regimens. They inhaled either placebo MDI and terbutaline NEB, or terbutaline MDI and placebo NEB for the first day of the acute response study and the first treatment fortnight. Crossover occurred on the second day of the acute response study and at the end of the first treatment fortnight.
Figure 1. Cumulative dose, FEV, response relationships (mean ± SE) for MDI and NEB in eight asthmatic patients.
Category: Airflow Obstruction
Tags: airflow obstruction, bronchodilator, lung, terbutaline