Canadian Neighbor Pharmacy: Clinical Pulmonary Hypertension
 VASODILAIAnON: ROLE OF ArACHIDONATE
VASODILAIAnON: ROLE OF ArACHIDONATE
Metabolites
There is accumulating evidence that products of the arachidonic acid cascade are involved in the control of the pulmonary circulation. Moncada and Vane suggested that the balance between thromboxane A (TXAJ, a potent vasoconstrictor, and prostacyclin (PGIJ, a potent vasodilator, may be more important than their absolute concentrations in regulating vascular tone. Recent studies emphasize the importance of the products of the lipoxygenase pathway of arachidonic acid, eg, leukotrienes, in modulating pulmonary vascular tone and in interacting with the cyclooxygenase pathway, ie, by inducing TXA release. Thus, it is possible that leukotrienes, to some extent, exert their actions via release of TXA.
Several mechanisms influence the relative proportion of constricting (thromboxane) and dilating (PCI2, PCE) eicosanoids produced when arachidonate is released by phospholipase. A basic mechanism is the amount of arachidonate released into the cyclooxygenase pathway: low levels (as occurs in the normal lung) lead preferentially to prostacyclin formation, the higher affinity biosynthetic pathway. Increasing concentrations of arachidonate lead to increasing expression of the thromboxane synthetase pathway. Several factors influence arachidonate availability. A common endpoint is deformation of the endothelial cell membrane, as occurs with contraction of subjacent smooth muscle cells in the course of vessel constriction, and cell surface shear forces related to flow through the vessel. Also, pharmacologic stimuli, egy angiotensin and bradykinin, induce prostaglandin release. It is generally held that the release of PGI induced by angiotensin serves to oppose angiotensin-induced pulmonary vasoconstriction. PGI is also released by bradykinin, and is believed to be a “second messenger” that enhances the vasodilating properties of the peptide.
Studies of endotoxemia and thromboembolism suggest a role for products of the arachidonate cascade in the pathogenesis of the early pulmonary hypertension often seen in acute lung injury. In experimental thromboembolism, greater increases in TXB2 (the stable breakdown product of TXAJ were observed than increases in 6-keto-PGF (the stable breakdown product of PGIJ. In endotoxemia, rises in TXBS correlate well with the increases in pulmonary pressure observed. Furthermore, pretreatment with TXAj inhibitors prevents the early pulmonary hypertension seen after endotoxin administration. Everything you need to order drugs is to check out the website of Canadian Neighbor Pharmacy.
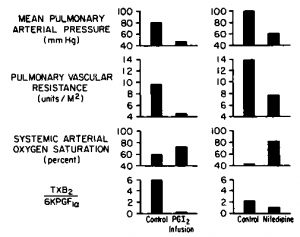
We studied the relation between pulmonary hemodynamics and the balance of TXA and PGI2 in a 17-month-old girl with severe pulmonary hypertension and clinical evidence of low-grade chronic lung injury of unknown cause. Resting measurements of prostanoids in unextracted plasma by radioimmunoassay showed an elevated TXB2to 6-keto-PGF^ ratio due to increased TXB2. Intravenous (IV) infusions ofPGI reduced mean pulmonary arterial pressure (from 80 to 47 mm Hg), increased cardiac output (from 3.4 to 4.0 L/min), increased systemic arterial oxygen saturation (from 60 to 72 percent), and decreased the TXB2 to 6-keto-PGF^ ra tio (from 6.0 to 0.2); mean systemic arterial pressure was unchanged (Fig 1). Not only did decreasing the TXB8 to 6-keto-PGF* ratio by infusing PGIj reduce pulmonary pressures and increase systemic arterial oxygen saturation, but also reducing the ratio by decreasing thromboxane levels with oral nifedipine and diltiazem had the same effect (6-keto-PGFte levels did not change with administration of nifedipine or diltiazem). On chronic diltiazem therapy for 12 months, mean pulmonary arterial pressure decreased to 27 mm Hg with no appreciable change in systemic arterial pressure or flow; systemic arterial oxygen saturation increased to 90 percent and the TXB2 to 6-keto-PGF ratio decreased to 1.0 due to a decrease in TXB8. Ibese findings support the hypothesis that the balance between TXA and PGI is important in regulating pulmonary vascular tone in some patients with pulmonary hypertension. Additionally, calcium-chan-nel blockers may work independently of changes in thromboxane. Hence, TXB8 may fell because pulmonary arterial pressure decreased, reducing surface shear forces (see below). The current data do not permit distinguishing between the relative contributions of these mechanisms.
tio (from 6.0 to 0.2); mean systemic arterial pressure was unchanged (Fig 1). Not only did decreasing the TXB8 to 6-keto-PGF* ratio by infusing PGIj reduce pulmonary pressures and increase systemic arterial oxygen saturation, but also reducing the ratio by decreasing thromboxane levels with oral nifedipine and diltiazem had the same effect (6-keto-PGFte levels did not change with administration of nifedipine or diltiazem). On chronic diltiazem therapy for 12 months, mean pulmonary arterial pressure decreased to 27 mm Hg with no appreciable change in systemic arterial pressure or flow; systemic arterial oxygen saturation increased to 90 percent and the TXB2 to 6-keto-PGF ratio decreased to 1.0 due to a decrease in TXB8. Ibese findings support the hypothesis that the balance between TXA and PGI is important in regulating pulmonary vascular tone in some patients with pulmonary hypertension. Additionally, calcium-chan-nel blockers may work independently of changes in thromboxane. Hence, TXB8 may fell because pulmonary arterial pressure decreased, reducing surface shear forces (see below). The current data do not permit distinguishing between the relative contributions of these mechanisms.
Interaction Between Mechanical and Metabolic Factors
Several studies suggest that arachidonate metabolism by pulmonary endothelial cells is altered by changes in flow dynamics, and that endothelial cell deformation in response to shear stress can increase the release of arachidonate, with normal healthy endothelial cells increasing PGI* release. These studies support the hypothesis offered by Rodbard that vascular shear stress controls vessel diameter. But since an increase in shear stress (from high flow and a large pressure drop across the vascular bed) can damage the endothelium, and injured endothelial cells may fail to release PGIs, the increased arachidonate released by the increase in shear stress may be diverted into the thromboxane synthetase pathway. This may lead to a vicious cycle of increased vasoconstriction, increased shear force, and increased damage, with perpetuation of the pulmonary hypertension.
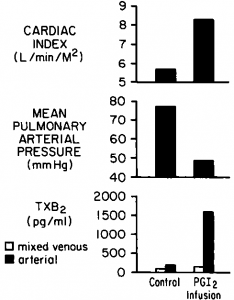
In three patients with idiopathic pulmonary hypertension, concentrations of TXB2 increased concomitantly with increases in pulmonary blood flow during IV infusions of PGI2. Aortic TXB2 concentrations were greater than pulmonary artery concentrations, suggesting pulmonary production of TXAg. In the data obtained from the patient depicted in Figure 2, a modest degree of pulmonary vasodilatation was achieved with PGIj infusion. The question posed by this patient is whether complete vasodilatation could have been achieved if thromboxane had not been released in the lung. We have observed other patients in whom PGI infusion increased pulmonary blood flow concomitant with increased pulmonary arterial pressure and with increased release of thromboxane. Failure of PGI* to vasodilate the pulmonary vascular bed in these patients may have resulted from the simultaneous release of thromboxane. It is possible that pulmonary hypertension can result from both decreased production of vasodilators such as PGI* (as in endothelial cell injury) and excess production of vasoconstrictors such as thromboxane. If excess production of vasoconstrictors is the cause, administration of exogenous vasodilators is unlikely to be of benefit unless the stimulus to increased release of vasoconstrictors can be overcome. These patients suggest that altered endothelial cell function (perhaps related to cell injury) may modify arachidonate metabolism and excerbate and perpetuate pulmonary hypertension in some patients. The pattern of arachidonate metabolites that we measured (increased TXB2 concentrations and/or the release of thromboxane when pulmonary blood flow increased) is consistent with changes in arachidonate metabolism seen in experimental models of lung injury.
Common to studies of several models of lung injury is damage to the endothelium, concomitant with decreased uptake of biogenic amines (serotonin and norepinephrine) and prostaglandins (PGE and PGF), decreased metabolism of vasoactive peptides (angiotensin I and bradykinin), and the release of TXAj and PGI2. Identification of the contribution to pulmonary vasoconstriction made by changes in the endothelial metabolism of vasoactive substances may lead to a more fundamental understanding of the control of the pulmonary circulation, and hence to more specific modes of therapy for clinical pulmonary hypertension.
Figure 1. Relation between pulmonary hemodynamics and the balance of TXA and PGI (measured as their stable metabolites, TXB2 and 6-keto-PGFfc, respectively) during IV PGl infusion or sublingual nifedipine in a 17 month-old-girl with idiopathic pulmonary hypertension. (Data modified from reference 30.)
Figure 2. Pulmonary release of TXBS concomitant with increased pulmonary blood flow during IV PGI* infusion in a five-year-old boy with idiopathic pulmonary hypertension.
Category: Pulmonary Hypertension
Tags: Clinical Pulmonary Hypertension, mechanical barrier, potent vasoconstrictor, pulmonary vascular tone, thromboxane